Zebrafish (Danio rerio)
MSc thesis at the University of Konstanz, Germany.
I explored the function of prion proteins (PrPs) during neural development in the zebrafish embryo.
I had two sub-projects: 1) the characterization of PrP transcriptional regulatory sequences in zebrafish,
and 2) the generation of transgenic zebrafish overexpressing fluorescent prion proteins in specific subsets of neurons.
The normal cellular function of the prion protein (PrP) has received relatively little attention because of its prominent role in neurodegenerative disorders. The human Creutzfeldt-Jakob disease, the bovine spongiform encephalopathy (BSE) and scrapie in sheep, are caused by the accumulation of an atypical isoform (“prion”) of the normal cellular prion protein, leading inevitable to death. The prion protein is highly conserved between several vertebrate species, which suggests an evolutionarily preserved role. Cellular function and disease likely represent two sides of the same coin and, in order to understand the molecular mechanism of prion diseases, it is essential to dissect also the natural function of PrP.
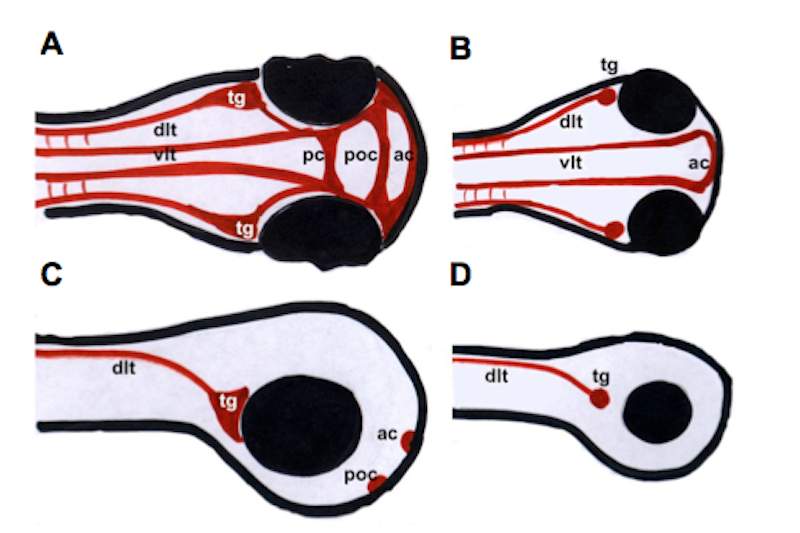
To this aim, the zebrafish (Danio rerio) was used in this study as an in vivo model. Due to an ancient genome duplication event, zebrafish possess two duplicated prion proteins (PrP-1 und -2), which exhibit an overall similar structure (repetitive domain, hydrophobic stretch and stable globular domain) to the mammalian prion protein. Nevertheless, they show unique expression patterns: whereas PrP-1 is expressed ubiquitously and early during zebrafish development, PrP-2 expression has a later peak limited to neuronal structures. These different expression patterns were probably adopted through modified regulation. In order to verify this assumption, the regulatory regions of PrP-1 and -2 were identified, amplified, cloned, PrP-transgenic zebrafish were generated and functionally characterized. Concordant expression of PrP-1 and -2 in trigeminal ganglion and Rohon-Beard neurons was driven by main regulatory elements that are probably shared. However, finer dissection and testing of the PrP-1 regulatory sequence showed that this sequence is likely to contain a repressor or silencing motif which stops expression during early zebrafish development. This is consistent with the early-restricted expression pattern of endogenous PrP-1.
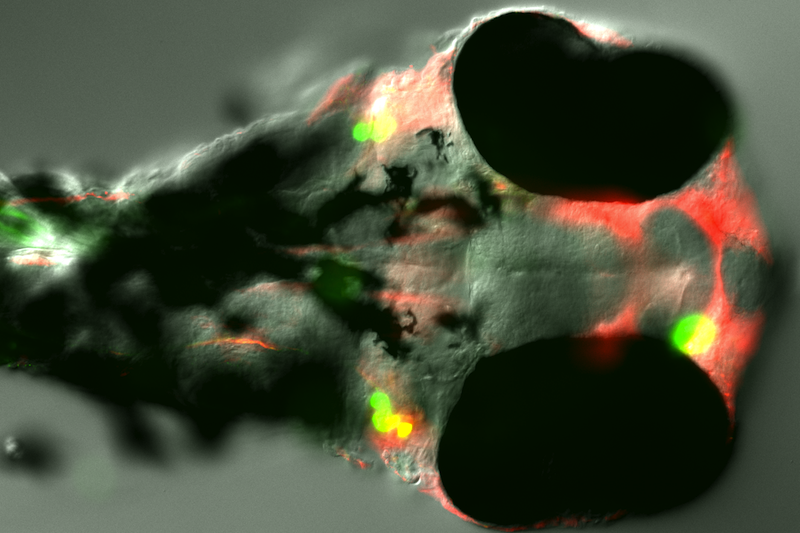
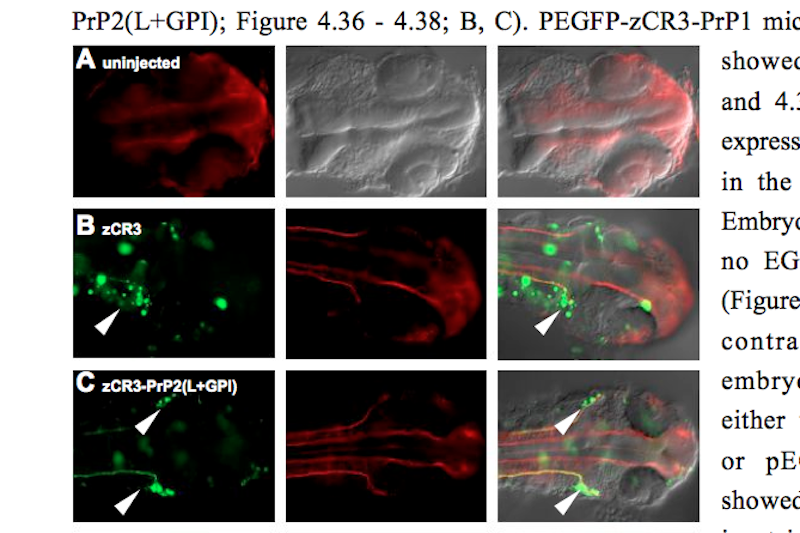
Furthermore, a method was developed to overexpress prion protein in zebrafish neurons. Specific neuronal enhancers (zCREST), which normally regulate the expression of the Islet-1 gene, were amplified, cloned upstream of EGFP-tagged prion proteins of several species and overexpressed in zebrafish neurons. Since PrP transgene expression could be observed at 24 hpf but not at 48 hpf it would appear that PrP overexpression is developmentally downregulated. PrP overexpressing neurons like trigeminal ganglion and Rohon-Beard neurons were analyzed using coinjections and immunohistochemistry but no major morphological defects could be identified. Whether the developmental downregulation of PrP involves mechanisms like degradation or apoptosis remains to be clarified.
Publications
 |
Barth JMI. Characterization of gene regulation and neuronal transgene expression of prion protein in zebrafish. Diploma thesis (2007) University of Konstanz. Download pdf. |