Fruit fly (Drosophila melanogaster)
PhD thesis at the ETH Zurich, Switzerland.
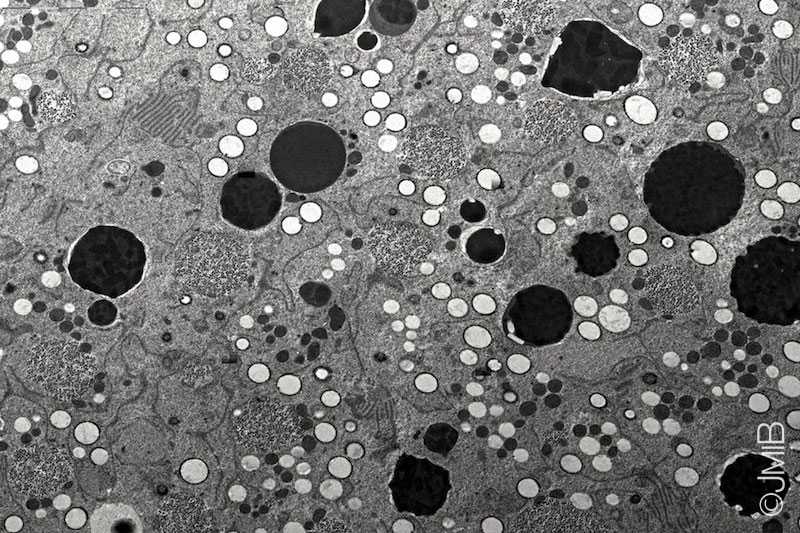
Autophagy is an evolutionarily conserved lysosomal degradation process promoting cell survival under several stresses, such as starvation.
Thereby, unneeded cytoplasmic components are broken down to reuse the resulting molecules for essential processes.
In small eukaryotes such as yeast, this is the major task of autophagy. However, in multicellular organisms, autophagic degradation is involved in many processes,
as for example in immunity and cellular remodeling. Furthermore, autophagy is also implicated in several human pathophysiologies, including neurodegeneration and cancer.
However, in most cases, the function and regulation of autophagy during development is still unclear, and new roles of autophagy are frequently revealed, highlighting the impact of autophagy research.
I provided novel findings that will help to clarify two major unsolved issues in the field of autophagy: The physiological implication of autophagy and the pathways guiding this function.
The major regulator of autophagy is the insulin/TOR signaling pathway. In Drosophila, insulin/TOR signaling influences the size and structure of ovaries, and nutrient depletion induces
autophagy in the fat body, but the role of nutrient signaling and autophagy during oogenesis remains unknown.
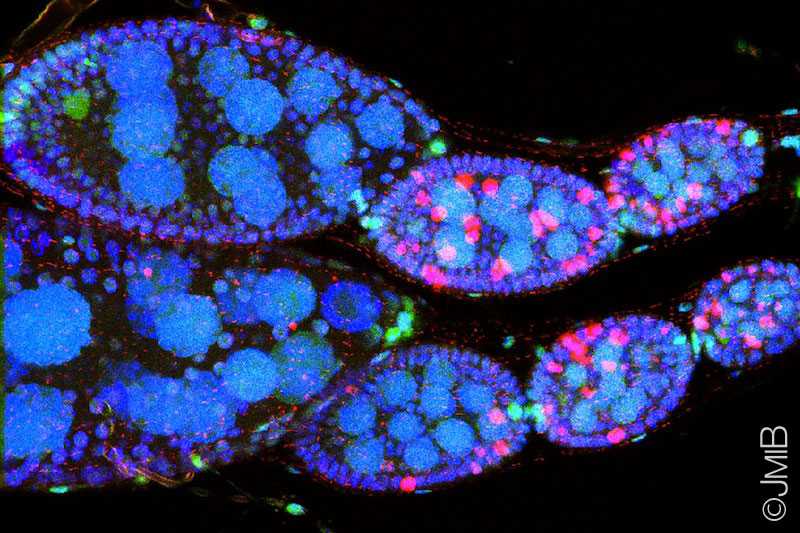
By establishing Drosophila ovaries as a novel model system to investigate autophagy, my work demonstrated that autophagy is induced by starvation in ovarian germ cells (GCs)
as well as in surrounding somatic follicle cells (FCs), which depends on a functional autophagic machinery.
Furthermore, I could show that the insulin/TOR pathway controls autophagy in these tissues. Additionally, the analysis of chimeric flies
indicated that autophagy is not required in GCs during oogenesis, but that loss of autophagy in FCs leads to developmental defects.
This mechanism depends on the cellular context, and defects are only present in a situation where FCs are mutant for autophagy, but GCs are wildtype.
Closer analysis of eggs covered by autophagy deficient FCs revealed several defects that have previously been described for flies exhibiting mutations in classical cell-cell
signaling pathways.
Egg morphogenesis depends on a tightly linked communication between FCs and GCs, and the regulation of
cell-cell signaling pathways has been demonstrated to involve endocytosis and endosomal trafficking.
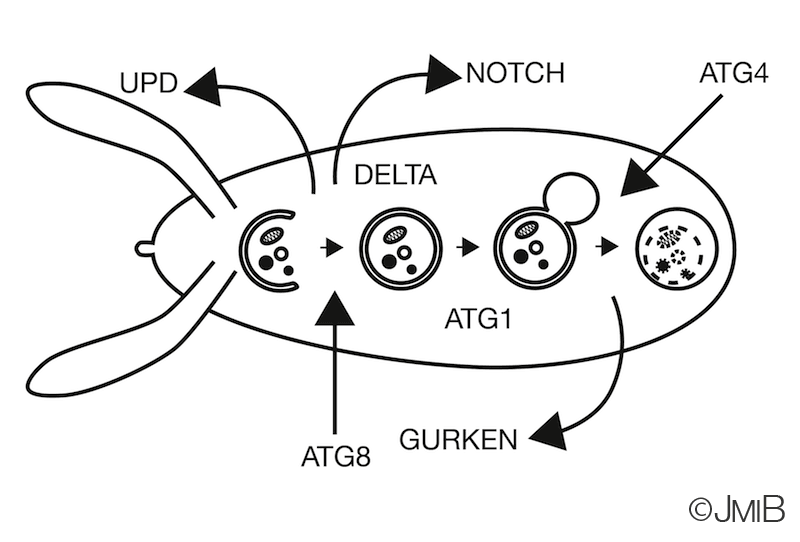
Since autophagy and endosomal pathways are known to intersect in order to target vesicles for lysosomal degradation, my work examined
whether autophagy modulates cell-cell signaling pathways. The posterior FCs that are important for cell-cell signaling events and egg
differentiation, were identified as the cell subpopulation responsible for autophagy dependent oogenesis defects. Furthermore, I could show
that loss of autophagy modulates Notch signaling in FCs.
Publications
 |
Barth JMI, Köhler K. How to Take Autophagy and Endocytosis up a Notch. BioMed Res Int (2014) 2014: 960803. doi:10.1155/2014/960803.
|
 |
Barth JMI, Hafen E, Köhler K. The lack of autophagy triggers precocious activation of Notch signaling during Drosophila oogenesis. BMC Dev Biol (2012) 12: 35. doi: 10.1186/1471-213X-12-35.
|
 |
Barth JMI. The role of autophagy during Drosophila oogenesis. PhD thesis (2011), ETH Zurich.
|
 |
Barth JMI, Szabad J, Hafen E, Köhler K. Autophagy in Drosophila ovaries is induced by starvation and is required for oogenesis. Cell Death Differ (2011) 18: 915-24. doi: 10.1038/cdd.2010.157.
|
 |
Köhler K, Brunner E, Guan XL, Boucke K, Greber UF, Mohanty S, Barth JMI, Wenk MR, Hafen E. A combined proteomic and genetic analysis identifies a role for the lipid desaturase Desat1 in starvation-induced autophagy in Drosophila. Autophagy (2009) 5 :980-90. doi: 10.4161/auto.5.7.9325.
|